- Published:
- 15 July 2024
- Author:
- Dr Euan Haynes and Dr Wendy Osborne
- Read time:
- 8 Mins
High-grade lymphomas have previously been associated with significant rates of relapse and refractory disease. However, innovative research has explored how the immune system can be harnessed to fight cancer, with high response rates and prolonged remissions.
What is CAR-T therapy?
CAR-T involves harvesting and genetically engineering a patient's own T-cells to target CD19 on lymphoma cells before reinfusing them. This treatment has been transformative in delivering high response rates and prolonged remissions; however, for patients who are unfit for CAR-T treatment or who relapse after CAR-T, the prognosis remains particularly poor.
Every year in the UK, around 5,500 people are diagnosed with diffuse large B-cell lymphoma (DLBCL), a highly aggressive type of non-Hodgkin lymphoma. First-line chemotherapy treatment is given with curative intent; however, around 30% of patients will have refractory disease or will relapse soon after initial treatment.
Historically, the prognoses associated with relapsed or refractory disease were dismal. If patients were fit, they could undergo an autologous stem cell transplant. Unfortunately, there is a significant cohort of relapsed or refractory patients whose lymphoma will not respond to autologous stem cell transplant or who are not fit enough to tolerate one. These patients had no effective treatment options and would often die in a short number of months.
Outcomes in the relapsed/refractory setting have seen substantial changes in recent years. Treatments have become available that harness the patient’s own immune system, rather than relying on chemotherapy. One such treatment is chimeric antigen receptor T-cell (CAR-T) therapy.A novel therapy
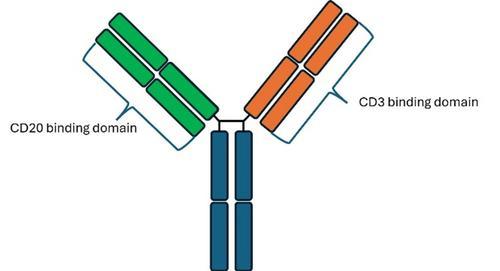
Figure 1: Schematic of epcoritamab with 1 CD3 and 1 CD20 binding domain.
In recent months, 2 bispecific antibodies, glofitamab and epcoritamab, have been approved by NICE in a third-line setting for patients with relapsed or refractory DLBCL. Bispecific antibodies have 2 arms that recognise separate antigens; glofitamab and epcoritamab recognise CD20 and CD3. The design of the 2 drugs does differ slightly, with epcoritamab having 1 CD3 binding site and 1 CD20 binding site (Figure 1), whereas glofitamab has a second additional CD20 binding site with 1 on either arm (Figure 2).
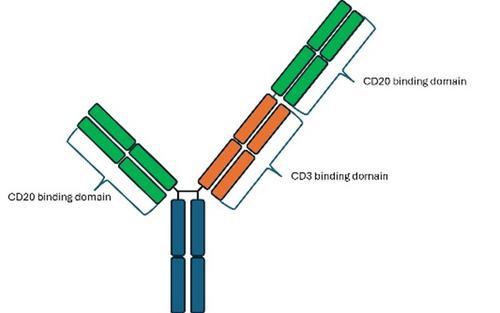
Figure 2: Schematic of glofitamab, which has 2 CD20 binding domains and 1 CD3 domain.
The CD20 arm binds to a lymphoma cell, while the CD3 arm binds to a host T-cell, then activates it and brings it into the lymphoma cell microenvironment. The activated T-cell then proliferates and releases cytokines, which initiate a cascade of immune activation (Figure 3), and also secretes perforin and granzyme, which directly induce cell lysis of the lymphoma cell.
During this process, bispecific antibodies use the patients’ T-cells to cause lymphoma cell death without causing the toxicity seen with chemotherapy. Approximately 40% of patients who would have had progressive lymphoma and often would have died within a few months can now obtain a complete response (CR) with this novel therapy.
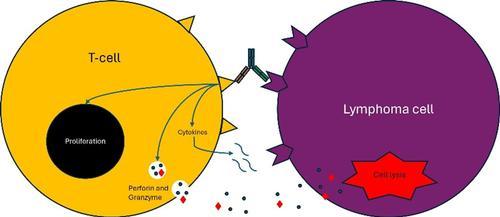
Figure 3: The CD3 and CD20 binding engages the T cell with the lymphoma B cell and causes immune activation.
Clinical impact
Clinical trial data for glofitamab and epcoritamab shows that 39% of patients with relapsed or refractory DLBCL who had already been failed by 2 lines of therapy achieved a CR.1,2 Approximately 66% of the patients who achieved a CR remained in a CR at 2 years. This cohort included many patients in a fourth- and fifth-line setting, so the responses may be even higher when given as a third line as per license.
One of the potential side effects of bispecific antibodies relates to the release of cytokines by the activated T-cells. This side effect, cytokine release syndrome (CRS), happens in the first 24–48 hours after the initial doses are given. There are mitigation strategies to reduce the incidence of CRS, including steroid premedication and a step-up dosing regimen.
Both glofitamab and epcoritamab only require 1 overnight stay in hospital, with the rest of the treatment usually given as an outpatient and patients describe an excellent quality of life while on treatment, unlike the quality of life patients describe with chemotherapy.
The wonder of the T-cell
Bispecific antibodies are ready-to-give, off-the-shelf medications that can be initiated on the same day that a patient is seen in clinic. They can be delivered in hospitals throughout the UK that have been trained in the management of CRS. This includes many district general hospitals, which improves the equity of access to effective treatments for patients.
The wonder of the T-cell has been clearly demonstrated by CAR-T data, which has longer follow-up than bispecific data, and has cured about 30–40% of patients who received CAR-T infusion. Bispecific antibodies are now an option for patients who relapse post-CAR-T or for some patients in whom the kinetics of their lymphoma is so aggressive that an immediate treatment is required.
To demonstrate the benefits of bispecific antibodies, here we report 2 case studies of DLBCL patients who had no other treatment options and would have died from high-grade lymphoma.
John had relapsed DLBCL and required third-line CAR-T cells, which obtained CR. In 2023, his DLBCL relapsed again; at this point, he initiated treatment with glofitamab, receiving 12 cycles in total. He achieved CR, which he remains in today. John describes his time on glofitamab as “like chalk and cheese compared to previous treatments,” adding that he “did not feel unwell at all while on treatment”.
Prestin was diagnosed with DLBCL in 2011 and was initially treated with immunochemotherapy. He subsequently suffered multiple relapses and underwent an autologous stem cell transplant in 2022. Unfortunately, though, he had refractory disease and so he received CAR-T later the same year. In 2023, his lymphoma relapsed again, at which point he began a treatment course of epcoritamab. After 3 cycles, he was in complete metabolic response and his lymphoma has remained in remission since. He describes the epcoritamab as “nothing like chemotherapy; it is much less harsh with no side effects – it is amazing”. His experience with epcoritamab was so different from his previous experiences, and he felt so well after starting treatment, that he did not believe that it was working.

Prestin and Dr Wendy Osborne, consultant haematologist.
Bispecific antibody treatments for DLBCL show great promise as novel therapies that use patients’ own T-cells to treat their cancer. Trials are already underway to investigate using bispecific antibody treatments in combination with chemotherapy or as a single agent in the first-line and second-line setting. These treatments have added to the growing arsenal of effective treatments for DLBCL. The treatment principle is one that can be applied to many other malignancies too, with huge potential to harness the wonder of the T-cell across other cancers.
References available on our website.
Return to the July 2024 Bulletin