- Published:
- 17 October 2024
- Author:
- Ellie Gilham and Diane Ashiru-Oredope
- Read time:
- 10 Mins
Emerging evidence suggests that vulnerable, deprived and minority populations face greater rates of antimicrobial resistance (AMR) and infection. This article details the work being done to identify the scope of these inequalities and what can be done to resolve them.
It has been estimated that 4.71 million (95% UI 4.23–5.19) deaths were associated with bacterial AMR in 2021, which includes 1.14 million (1.00–1.28) deaths attributable to bacterial AMR.1 This is expected to increase to 1.91 million deaths annually by 2050.2 These infections are also associated with a significant cost to the global economy of approximately $100 trillion annually.2
A Global Action Plan was published by the World Health Organization in 2015. 193 countries subsequently signed a UN declaration in 2016 to address the root causes of antimicrobial resistance (AMR) within human health, animal health and agriculture by strengthening regulation of antimicrobials, improving knowledge and awareness of AMR and promoting best practices.3 The declaration also promoted the use of innovative approaches by using alternatives to antimicrobials and new technologies for diagnosis and vaccines. Multiple countries have now published and implemented national action plans (NAPs) to tackle AMR.4
There is emerging evidence of increased rates of infection and resistant infection,5 as well as higher levels of antimicrobial exposure, in individuals with factors commonly associated with health inequalities.6
The prevalence of hepatitis C and B, tuberculosis (TB) and respiratory conditions that are likely to lead to increased rates of respiratory infection, such as asthma, is higher in homeless populations, individuals with substance use disorders, sex workers and individuals in contact with the justice system.7 Furthermore, people from ethnic minorities, deprived individuals and inclusion health groups, such as vulnerable migrants (including asylum seekers and refugees, unaccompanied asylum-seeking children, people who have been trafficked, undocumented migrants who are living in the UK with no legal status and low-paid migrant workers) have consistently been shown to be at higher risk of infections, including TB, sexually transmitted infections and methicillin-sensitive Staphylococcus aureus (MSSA).8–12
Higher levels of antibiotic use have also been identified within groups such as older adults, especially those in residential care and those living in areas with higher levels of deprivation. Furthermore, vulnerable migrants often rely on alternative routes of antibiotic supply owing to barriers faced in accessing healthcare within the UK. This may lead to higher rates of inappropriate antibiotic use within this population.6
The UK Health Security Agency (UKHSA) has conducted multiple health campaigns to raise public awareness of AMR, improve public knowledge of correct antibiotic use and support healthcare professionals to prescribe appropriately, including Antibiotic Guardian,13 Keep Antibiotics Working,14 e-Bug15 and TARGET.16 While campaign evaluations have outlined the effectiveness of these programmes at increasing public engagement, awareness and knowledge, and at supporting healthcare professionals, it was unclear whether they were having an equitable impact across all demographics.
This led to the evaluation of public health campaigns using a health equity assessment tool (HEAT).17 The campaigns have demonstrated equality and diversity in terms of their reach, based on the Equality Act 2010, as they were accessible to individuals of different ages, sexes, races and other protected characteristics. Notably, this includes the translation of website materials into over 30 languages and reaching individuals in 122 countries.
It was noted, however, that several of the protected characteristics were not applicable across all campaigns; for example, marriage/civil partnership would not be relevant for the e-Bug campaign, which is aimed at children. The continuous development of resources with collaboration from a variety of diverse user groups would aid future campaign reach. The use of the HEAT tool has demonstrated an easy and cost-effective way to assess reach to diverse groups, providing assurance that campaigns and resources do not increase existing health inequalities, and would be a useful addition to all antimicrobial stewardship and public health campaigns.17
Following evaluation of campaigns using the HEAT tool, a health-inequality-specific workstream was established in 2019 within the Healthcare-Associated Infection and AMR division of UKHSA. Objectives and key performance indicators (KPIs) were subsequently developed with the aim of embedding a systematic approach to reducing health inequalities in AMR (Figure 1).
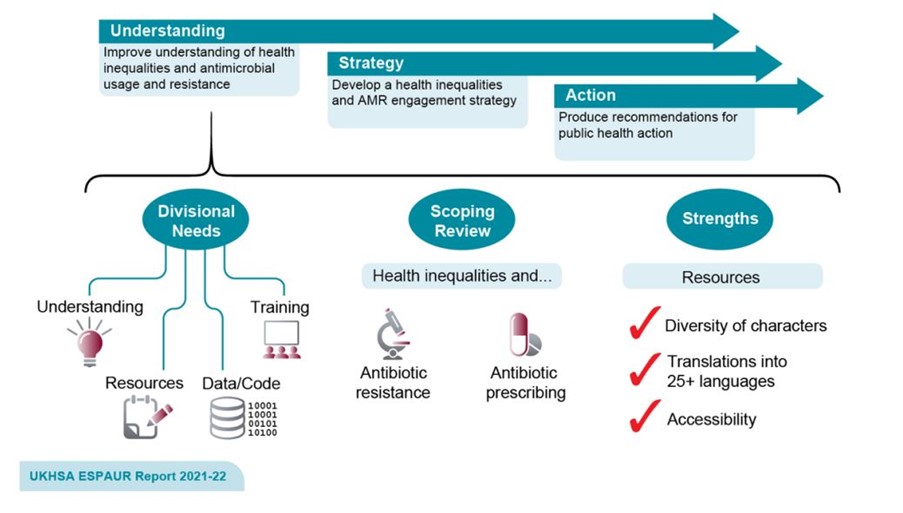
Figure 1: Overview of UKHSA’s workstream to address health inequalities across the work of the UKHSA Healthcare-Associated Infection, Fungal, Antimicrobial Resistance, Antimicrobial Usage and Sepsis Division.
One such KPI was to improve AMR and antimicrobial consumption (AMC) surveillance to understand the burden and trends of AMR and AMC for Core20PLUS5 populations (Figure 2). Subsequently, levels of data reporting on health inequalities and AMR were increased within the annual English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) report.
Core20PLUS5 is a national approach used by NHS England to reduce health inequalities at a national and systemic level18 (Figure 2). Core20 refers to the most deprived 20% of the national population, defined by the national Index of Multiple Deprivation.19 PLUS population groups refer to inclusion health groups (people experiencing homelessness, drug and alcohol dependence, vulnerable migrants, Gypsy, Roma and Traveller communities, sex workers, people in contact with the justice system, victims of modern slavery), groups that share protected characteristics defined by the Equality Act 2010 (age, gender reassignment, marital status, pregnancy, disability, race, religion, sex and sexual orientation), as well as ethnic minorities, people with a learning disability or autism, and people with multiple long-term health conditions.
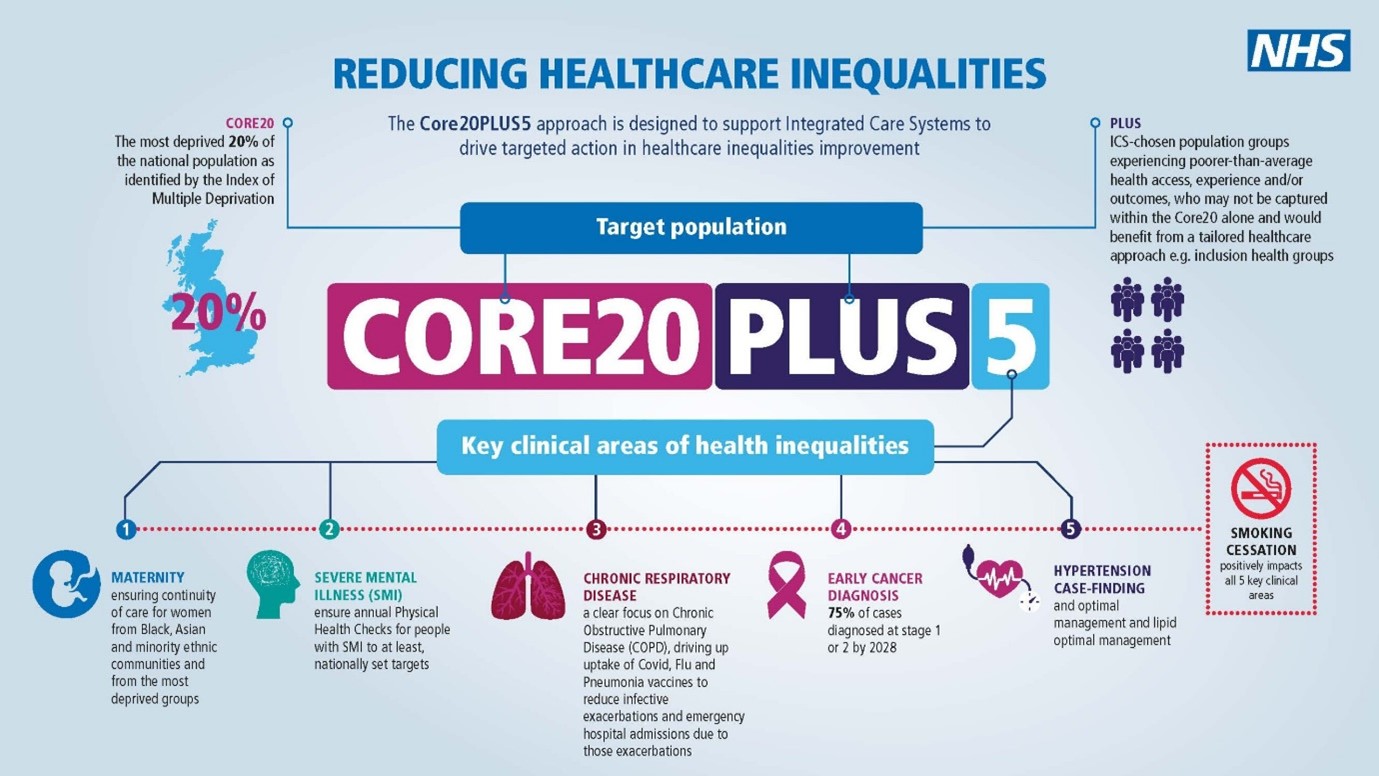
Figure 2: NHS England CORE20PLUS5 – an approach to reducing healthcare inequalities.18
Data published in the 2022–2023 ESPAUR report highlights the difference in AMR burden seen in populations that have a number of factors commonly associated with health inequalities, including deprivation, ethnicity, age and regional variation (Tables 1–3 and Figure 3). Rates of resistant bloodstream infections are 41% higher in the most deprived compared to the least deprived groups (33 versus 24.3 infections per 100,000 population).20 Furthermore, this percentage difference has increased by 7% from 2019, while the difference in rate of bloodstream infections between the most and least deprived groups has remained constant over this time period.
The 2022–2023 ESPAUR report also highlights a higher burden of AMR in bloodstream infections within Asian and British Asian compared to White individuals (34.6 versus 18.7%). The rate of carbapenemase-producing gram-negative bacteria infection was also higher among Asian and British Asian ethnic groups (7.7 per 100,000 minority ethnicity population versus 4.1 per 100,000 for White populations).
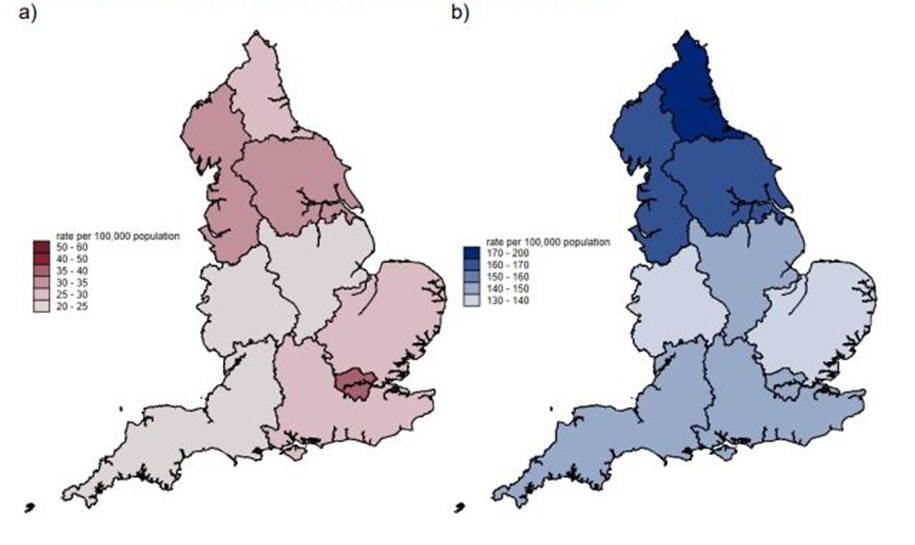
Figure 3. Regional variation in rate per 100,000 population. (a) The estimated burden of AMR. (b) The estimated numbers of bloodstream infections in England in 2022.
AMR burden was also shown to vary by age. The rate of resistant bloodstream infections was highest in >74-year-olds (157.0 per 100,000) and lowest in the 5–9-year-old age group (4.8 per 100,000). Finally, regional variation in AMR was reported, with the London region reporting the highest AMR burden rate (39.2 per 100,000 population) followed by the Northwest (32.9 per 100,000 population). The lowest AMR burden rate (from bloodstream infections) was recorded in the Southwest (22.8 per 100,000 population). This data echoes other findings in the literature that demonstrate higher rates of resistant infections in minority ethnic populations,21–24 with economic deprivation25,26 and within inclusion health groups, such as vulnerable migrants.27
Table 1
| Ethnic group | Rate of BSI per 100,000 ethnic population (n) | Rate resistant per 100,000 ethnic population (n) | Percent resistant (95% confidence intervals) |
| White | 150.7 (68,983) | 28.1 (12,870) | 18.7% (18.4 to 18.9) |
| Asian or Asian British | 77.1 (4,185) |
26.7 (1,450) |
34.6% (33.2 to 36.1) |
| Black African, Black Caribbean or Black British | 94.0 (2,240) | 24.0 (570) | 25.5% (23.7 to 27.3) |
| Mixed or multiple ethnic groups | 33.4 (558) | 6.4 (107) | 19.2% (16.0 to 22.5) |
| Any other ethnic group | 25.3 (311) | 4.7 (58) | 18.7% (14.3 to 23.0) |
| Not known or not stated | N/A (1,262) | N/A (190) | 15.0% (13.1 to 17.0) |
|
* 4,982 (6.0%) BSI episodes could not be linked to obtain ethnic group information. The percentage resistant in this group was 18.3% (n=911). |
|||
Table 2
| IMD quintile | Rate of BSI per 100,000 population (n) | Rate of BSI per 100,000 population (n) | Percent resistant (95% confidence intervals) |
| 1 (most deprived) | 163.3 (18,455) | 33.0 (3,729) | 20.2% (19.6 to 20.8) |
| 2 | 146.8 (17,078) | 29.2 (3,397) | 19.9% (19.3 to 20.5) |
| 3 | 142.8 (16,399) | 28.6 (3,288) | 20.0% (19.4 to 20.7) |
| 4 | 135.0 (15,090) | 28.6 (3,288) | 18.9% (18.2 to 19.5) |
| 5 (least deprived) | 125.9 (13,793) | 23.4 (2,567) | 18.6% (18.0 to 19.3) |
Table 3
| Age group (years) | Rate of priority BSI per 100,000 population (n) | Rate resistant per 100,000 population (n) | Percent resistant (95% confidence intervals) |
| Under 1 | 300.7 (1,742) | 46.5 (269) | 15.5% (13.8 to 17.2) |
| 1–4 | 37.4 (928) | 4.8 (119) | 12.8% (10.6 to 14.9) |
| 5–9 | 16.0 (536) | 2.4 (80) | 15.0% (11.9 to 18.0) |
| 10–14 | 11.1 (381) | 1.8 (60) | 15.9% (12.2 to 19.5) |
| 15–44 | 35.8 (7,755) | 6.4 (1,382) | 17.8% (17.0 to 18.7) |
| 45–64 | 120.3 (17,541) | 23.2 (3,377) | 19.2% (18.7 to 19.8) |
| 65–74 | 297.2 (16,543) | 64.2 (3,574) | 21.6% (21.0 to 22.2) |
| Over 74 | 767.6 (37,624) | 157.0 (7,693) | 20.4% (20.0 to 20.9) |
| Unknown | N/A (43) | N/A (22) | 52.9% (37.9 to 67.9) |
Tackling health inequalities related to AMR has now been included as an outcome with associated commitments in the recently published 2024–2029 National Action Plan for AMR,28 which states that, "by 2029, the UK targets interventions and associated funding where there is the most burden from AMR, where it will have the greatest impact in controlling AMR and where it will be cost-effective, including targeting specific regions, population groups and settings if appropriate".28
Future work will focus on improving access to and dissemination of data which reports on differences in infection incidence, AMR and antimicrobial use in the context of health inequalities. This will allow for more bespoke epidemiological analyses and inform interventions for marginalised, disadvantaged, vulnerable and high-risk populations.
Furthermore, additional and tailored support can only be provided to vulnerable groups if teams in local government and organisations, such as local authorities and NHS trusts, are knowledgeable of ways to address potential inequalities that have been identified in their area. Therefore, future work will also look to develop a toolkit that collates evidence-based resources for identifying and addressing inequalities in healthcare access, infection incidence, clinical outcomes, vaccine uptake and antimicrobial exposure at a local community level.
In addition to the use of data to inform interventions that target certain points in the patient infection pathway, other interventions that can be adapted to local needs will also be identified from the published literature. Work regarding this is already underway, with a series of ongoing rapid reviews that look to determine AMR burden and identify interventions to tackle AMR in inclusion health groups. There are currently reviews on populations in prisons (OSF registration: https://doi.org/10.17605/OSF.IO/XHCFJ), sex workers, adults in social care (PROSPERO 2024: CRD42024494928) and people who use drugs (PROSPERO 2024: CRD42024561876).
In conclusion, while AMR itself is complex, with multiple factors contributing to increases seen in resistant infections, the extent to which vulnerable and marginalised communities are disproportionately affected is only just being understood. Significant progress has been made in recent years to understand the effect of health inequalities on AMR burden. However, further work is needed to determine whether these differences are being seen across all groups which suffer from health inequalities, to understand the driving factors for the differences in infection incidence, AMR and antimicrobial use within these populations and to develop and implement effective interventions.
References available on our website.
Return to October 2024 Bulletin
Read next
Tackling ethnic inequalities in precision and genomic medicine
17 October 2024
Inequalities in cancer – an unequal burden
17 October 2024
Global antimicrobial resistance webinar series
17 October 2024
Antimicrobial resistance at International Pathology Day
17 October 2024