- Published:
- 15 July 2024
- Author:
- Professor Justin Stebbing
- Read time:
- 7 Mins
This article highlights the current research at the forefront of cancer treatments – the novel nucleic acid vaccines that provide a personalised immune response against tumours.
What are mRNA cancer vaccines?
mRNA vaccines are a type of nucleic-acid-based vaccine that contains mRNA. These vaccines can encode antigens (such as tumour-associated antigens (TAAs) or tumour neoantigens) that can be delivered to the immune system via different delivery mediums.1 The principles of mRNA vaccines tend to involve:
- introducing mRNA into the body via an appropriate delivery medium, often lipid nanoparticles
- designing and sequencing mRNA to carry instructions for cells to produce the appropriate TAAs and neoantigens
- activating an immune response via the antigens produced
- tailored treatment with personalised mRNA vaccines.1,2
Definitions
- Tumour neoantigens: self-antigens generated by tumours because of genomic mutations. Completely unique and tumour-specific.
- Tumour-associated antigens (TAAs): elevated levels on tumour cells that are also expressed at lower levels on healthy cells.
- Tumour-specific antigens (TSAs): found only on cancer cells, not on healthy cells.
- Immunogenicity: ability of therapeutic protein products to stimulate an immune response.
Tumour samples from an individual are taken and are sequenced to identify TAAs or tumour neoantigens.2 Once the most immunogenic antigens are selected, they can be designed into mRNA sequences that can encode into the specific antigens selected.2 The mRNA sequence is usually encapsulated by lipid nanoparticles, which are then injected into the patient; it is then translated to create the TAAs and tumour neoantigens that stimulate an immune response.
Unlike for preventative vaccines, which rely on B cells producing antibodies via a humoral response,2 a potent T-cell response is needed to attack the tumour in therapeutic cancer vaccines. In some ways this is different to the mRNA vaccines used during the COVID-19 pandemic, which relied mainly on a B-cell antibody response.
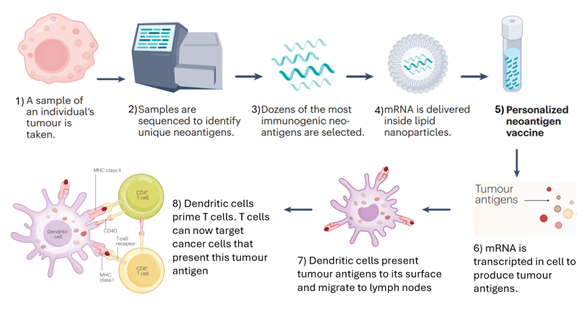
Figure 1: A basic overview of how an mRNA cancer vaccine is created and how they work. Adapted from Drew (2024).2
Physicians have long attempted to harness the power of the immune system to treat cancer. We now have the technology that enables us to vaccinate patients with mRNA that can be encoded to TAAs and tumour neoantigens. This technique has the advantage of allowing personalised vaccinations to be produced, as we can target specific TAAs and tumour neoantigens via tumour exome analyses of individual patients’ tumour samples.3
It is useful to note that mRNA vaccines have the benefit of being able to allow multiple antigens to be administered in 1 immunisation. This means that it is possible to target multiple antigens at once, depending on an individual’s unique tumour profile.1
Dendritic cell vaccines
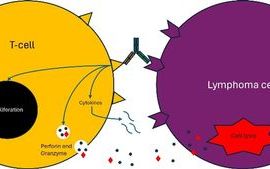
Another use is for mRNA vaccines is in cellular therapies that transfect patient-derived cells, e.g. dendritic cells in vitro.3 Dendritic cells are antigen-presenting cells (APCs), which can prime T cells to recognise a particular antigen that stimulates an adaptive immune response.3 Instead of being exposed to proteins, dendritic cells can be exposed to mRNA encoding TAAs and neoantigens, which allows them to produce the tumour antigens themselves and present these antigens on their surfaces.3 This, in turn, primes T cells so that the immune system can recognise the antigens and attack cancer cells that express these antigens.3
An advantage of this approach is that it reduces the risk of autoimmunity. The treatment avoids growing patient-specific tumours,3,4 as patient-specific tumours contain the patients’ normal self-antigens that can be recognised by the immune system. A limitation to using mRNA encoding into TAAs is that we do not know the TAAs for many cancers; this gap in knowledge is problematic, as we need to identify the suitable TAAs needed when designing mRNA vaccines.3
CAR-T cell therapy
mRNA cancer vaccines are also useful in chimeric antigen receptor-T (CAR-T) cell therapy. CAR-T uses patient-derived T cells, which are genetically engineered to express receptors that target specific TAAs and, therefore, can attack specific tumours.3 Here, mRNA can be used to help engineer these T cells through methods such as mRNA electroporation. A phase I trial has been initiated with 2 patients with solid malignancies (1 with advanced mesothelioma and the other with metastatic pancreatic cancer).5
Immune checkpoint inhibitors
Among the most exciting use cases to date, mRNA cancer vaccines have demonstrated dramatic improvements in outcomes when combined with immune checkpoint blockade in patients with stage III melanoma. For example, a randomised phase IIb trial of a personalised mRNA neoantigen vaccine with an immune checkpoint inhibitor (pembrolizumab) for high-risk melanoma reported improved recurrence-free survival compared to pembrolizumab alone.6
In the overall intention-to-treat population, adjuvant treatment with mRNA-4157 (V940) in combination with pembrolizumab demonstrated a statistically significant and clinically meaningful improvement in distant-metastasis-free survival compared to pembrolizumab (i.e. routine care) alone. The combined treatment reduced the risk of developing distant metastasis or death by 65% (HR = 0.347 [95% CI: 0.145–0.828]; one-sided p value = 0.0063).
A phase I trial of a personalised adjuvant mRNA neoantigen vaccine for pancreatic cancer reported that those who responded to the vaccine in terms of a T-cell response had a longer median recurrence-free survival compared to non-responders to the vaccine.7
The future of mRNA cancer vaccines requires an analysis of tumour cell samples from individuals and, subsequently, sequencing and designing the vaccines and treating patients with them. Again, this personalisation may mean that we can treat cancer patients more effectively by targeting antigens unique to each patient’s specific tumour profile.
Thus far, mRNA cancer vaccines seem well tolerated. As mRNA vaccines target specific TAAs and tumour neoantigens, they may have fewer side effects compared to current treatments, such as chemotherapy or radiotherapy. This would, therefore, help to improve the quality of life of cancer patients and increase their quantity of life and cure rate.
Clinical applications in the UK
The UK government has partnered with BioNTech, the German company known for its work with Pfizer on mRNA vaccines for SARS-CoV-2 during the COVID-19 pandemic.8 This long-term partnership is reported to potentially allow access to precision cancer treatments for up to 10,000 patients in the UK by 2030.8 This partnership aims to establish the Cancer Vaccine Launch Pad, whereby potentially eligible cancer patients are identified and a database is created for those who are suitable to participate in personalised vaccine trials.8
New facilities and staff are included in the plans to support this project, such as new laboratories in Cambridge.8 NHS patients are currently being enrolled for a phase II BioNTech mRNA cancer vaccine trial for high-risk resected colorectal cancer at University Hospitals Birmingham NHS Foundation Trust; other sites are expected to enrol most patients after 2026.8
Future studies
As mentioned above, the phase IIb study of a personalised mRNA neoantigen vaccine (mRNA-4157) plus pembrolizumab versus pembrolizumab monotherapy for resected high-risk melanoma reported that patients with the combination therapy had a 49% reduction in risk of recurrence or death after 3 years compared with the standard monotherapy treatment.6,9–11 At the American Society of Clinical Oncology meeting in Chicago, June 2024, Moderna presented a planned follow-up of their phase 2b study at 34.9 months, also showing a 62% reduction in risk of distant metastases. Following these promising results, the trial has now been expanded to a phase III global clinical trial with an aim to recruit 1,100 people.10,11 Depending on the success of the trial, it is possible that we may see mRNA cancer vaccines being offered as a potential treatment option in the future, especially in the case of patients who have high-risk resected melanoma.
Moderna has already discussed filing with the US Food and Drug Administration (FDA), owing to their impressive data in the phase IIb study for melanoma patients, with the aim to decrease the recurrence rate after surgery. It is not known whether the FDA will give the product an accelerated approval ahead of the results from the phase III study, which will take a few years. If they do, we could see the treatment being given routinely to patients in the US next year; it is possible that European regulators may follow suit.
Large trials, in addition to standard of care, have commenced in common tumour types such as non-small cell lung cancer. A key question in the future is whether mRNA cancer vaccines have monotherapy clinical activity or require use in combination. Either way, these are exciting times. My personal view is that mRNA vaccines will become part of standard of care in years to come, representing a completely new therapeutic modality.
References available on our website.
Return to the July 2024 Bulletin