The specialties of medical microbiology and virology have been at the forefront of major public health breakthroughs, from the development and testing of vaccines to the characterisation of viruses such as hepatitis and advances in hand hygiene.
Infection is an ever-evolving specialty, as new pathogens that may present diagnostic and treatment challenges are constantly emerging with possibly devastating consequences in a naive population. At the same time, we continue to face the challenges of prevention, diagnosis and treatment of infections in patients with complex clinical needs and seek new treatments where the immune system is impaired, such as for cancer or autoimmune diseases.
Infection is an ever-evolving specialty, as new pathogens that may present diagnostic and treatment challenges are constantly emerging with possibly devastating consequences in a naive population.
Infections may be acquired in the community, in association with healthcare or travel. Without prompt diagnosis and management, many infections are associated with considerable mortality and morbidity and the ability to transmit to other persons.
Global awareness of infection has been increasing over recent years, with infections such as Clostridium difficile, methicillin-resistant Staphylococcus aureus, avian influenza, Middle Eastern respiratory syndrome coronavirus, Ebola and SARS-CoV-2 all making headlines across the world.
Even more commonly encountered and less exotic infections (e.g. tuberculosis, hospital-associated infections, urinary tract pathogens or chest pathogens) can present complex challenges that require the expertise of an infection specialist. One example is meningitis caused by Neisseria meningitidis, for which the introduction of antibiotics in the community for suspected meningococcal disease has resulted in a huge reduction in mortality and morbidity. However, giving antimicrobials prior to taking samples to look for infection (e.g. cerebrospinal fluid and blood cultures) means that the chances of obtaining a positive culture result are massively reduced as well as fostering microbial drug resistance.
Multidrug resistance is an increasing problem, therefore antimicrobial stewardship and infection prevention and control (IPC) remain cornerstones of an infection service.The Medical Microbiology Specialty Advisory Committee (SAC) and Medical Virology SAC merged in 2019, with the inaugural joint meeting being held on 1 November 2019. The role of the SAC is varied and it holds accountability for strategy, standards and best practice, specialty engagement, and supports other committee and College activities.
Key achievements over the past 60 years
Vaccines
The rapid advancement in the development of vaccines has led to a massive worldwide reduction in mortality and morbidity caused by previously life-threatening infections. The importance of vaccines has been especially highlighted in recent years, with widespread use of the influenza vaccine following the H1N1 pandemic and, more recently, the use of vaccines against SARS-CoV-2 and its variants. In 1980, the World Health Organization (WHO) declared that smallpox had been globally eliminated. The introduction of the polio vaccine means that, as of 2020, only Pakistan and Afghanistan are recognised as countries with endemic spread of polio. Other vaccines anticipated to make a global impact include vaccines for Ebola, malaria and Lassa.
The meningococcus serogroup C vaccine, which has been available for several years, and, more recently, the development of the quadrivalent meningococcal vaccine, have resulted in a reduction in meningitis in the UK population. The UK immunisation schedule has also delivered a reduction in the mortality and morbidity associated with diphtheria, tetanus, pertussis, measles, hepatitis B, rubella, mumps and Streptococcus pneumoniae. Other vaccines of note include the HPV vaccine, which has led to a reduction in the incidence of cervical cancer, and the rotavirus vaccine, reducing hospital admissions in developed countries and reducing the high mortality seen in infants in developing countries. Infant mortality and morbidity caused by sepsis and meningitis from Haemophilus influenzae serogroup B has been drastically reduced thanks to the development of the Hib vaccine. Vaccination against hepatitis A is also widely proscribed for risk groups and travellers to endemic countries.
Molecular testing
The advent of molecular methods for the detection of microbial pathogens and the presence of anti-microbial resistance genes have greatly advanced infection diagnostics with an impact on antimicrobial stewardship, as well as the reduction in mortality and morbidity of potentially life-threatening infections.
The widespread availability of viral load testing has been key in the identification and treatment of disease caused by viral infections such as hepatitis B, cytomegalovirus, HIV and hepatitis C.
PCR and other molecular amplification methods have helped us to detect rapidly and identify infections in sick patients to select the most appropriate treatments for them; these techniques also help screening to prevent transmission of disease such as in blood products. Automation of these molecular methods has been key in supporting the ever-increasing demands of higher and higher numbers of samples that need to be tested as quickly and with as much information as possible for appropriate treatment and infection control.
The widespread availability of viral load testing has been key in the identification and treatment of disease caused by viral infections such as hepatitis B, cytomegalovirus, HIV and hepatitis C.
Hepatitis C
The characterisation of hepatitis C virus (HCV) is one of the major triumphs of modern science and medicine. Originally investigated as a cause of post-transfusion hepatitis, the nature of the causative agent evaded all classical virological attempts at characterisation. However, a blind cDNA immunoscreening approach – whereby the protein product of cloned nucleic acid was signalled by antibody probing with serum from a patient with active disease – yielded a fragment of the genome. This facilitated entire genome sequencing, which was published in 1989.1 By 2014, the function of all proteins encoded within the genome had been elucidated, and small molecules designed to inhibit those functions were tested in a myriad of clinical trials.
Owing to the success of directly acting antiviral agents, over 95% of treated patients are cured of their infection within 12 weeks. Thus, within a period of 25 years we progressed from not knowing the pathogen to almost complete cure. An estimated 72 million people infected with HCV will benefit from this.
In 2015, WHO announced a strategy for the elimination of HCV as a public health concern. This outstanding achievement was recognised appropriately in 2020 by the award of the Nobel Prize for Medicine or Physiology to three of the key players – Harvey Alter, Michael Houghton and Charles Rice.
Biomarkers
The concept of a circulating biomarker for the diagnosis of invasive aspergillosis in immune-compromised patients by ELISA was first described in 1976 by the University of Birmingham mycology research group,2 and later characterised as galactomannan. This is a key diagnostic test for pulmonary aspergillosis in haematology patients and, more recently, in COVID-associated pulmonary aspergillosis. The use of biomarkers for the diagnosis of infection is a rapidly evolving one that will greatly enhance the infection diagnostics repertoire.
Monoclonal antibodies
It was known that myeloma cells could cause a gammopathy through the excessive excretion of antibody molecules. Milstein in Cambridge visited by Köhler from Basel worked on developing hybrid cells, now called hybridomas, by fusing myeloma cells with B cells. In 1975, Milstein and Köhler described immortalised hybridomas secreting anti-bodies of known specificity, resulting in the 1984 Nobel prize. It provided access to specific diagnostically important reagents, which previously would have been generated by hyper immunisation of animals, probably rabbits.
To a great extent, monoclonal reagents have led to routine detection of microbial antigens for diagnostic purposes, e.g. in lateral flow tests for diagnosing SARS-CoV-2 infection.
The description of humanisation of such mono-clonal antibodies by Greg Winter in 1988 made it possible to use such humanised antibodies as therapeutic agents. To a great extent, monoclonal reagents have led to routine detection of microbial antigens for diagnostic purposes, e.g. in lateral flow tests for diagnosing SARS-CoV-2 infection. The only downside of such reagents is that the epitopes recognised are very tightly defined by amino acid sequence, losing reactivity through viral evolution.
Oral microbiology
The specialty of oral microbiology was established in late 1970s to provide dentists access to medical microbiology training. This ensured skills, knowledge and expertise in diagnostics and management of infection, antimicrobial stewardship and IPC in general.
Advances in hand hygiene
It was Ignaz Semmelweis who discovered the role of handwashing in reducing the spread of infection in Hungary in 1847. Hand hygiene is a crucial and effective measure in preventing healthcare-associated infections (HCAIs) and is considered the cornerstone of good IPC practice. One of the greatest achievements in medical microbiology and IPC in the last 60 years is the development of the Ayliffe six-step handwashing technique. Developed by Professor Graham Ayliffe and his team, it is widely adopted in clinical practice and endorsed by the WHO.
Hand hygiene is a key component of the aseptic non touch technique (ANTT) framework that was developed in the 1990s. This is a framework for all invasive procedures, from surgery to mainte-nance of invasive lines. Use of ANTT has resulted in a reduction in HCAIs and post-surgical infections.
Now, alcohol-based hand rubs are widely used and hand decontamination and good hand hygiene is no longer the sole remit of health-care workers; the general public have learned to sing Happy Birthday while decontaminating their hands.
Key challenges facing the specialty
Reconfiguration and privatisation
The last ten years have seen increasing demands placed on the NHS as a whole, amid numerous challenges owing to reconfiguration and reorganisation of the NHS and associated regulatory organisations such as NHS Improvement (NHSI) and the NHS England Care Quality Commission (CQC).
In the medical microbiology and virology specialties, there is the increasing threat of priva-tisation, already in place in some areas as a result of NHSI’s pathology reconfiguration. This places private laboratories in direct competition with NHS trust-based laboratories.
Workforce
Without doubt, one of the biggest challenges is a shortage of workforce. Workforce shortages have been brought on by Agenda for Change, Brexit and public sector pay freezes, as well as loss of bursaries for nurses. Infection services have been chronically understaffed. Pathology reconfiguration and an increasing demand for services, such as involvement in multidisciplinary team meetings and clinical consultations, as well as the combined infection training and shape of training, have only served to highlight the large gaps in workforce. This has been brought to the fore by the pandemic, which has shown how understaffed the NHS is in terms of workforce at the ward and laboratory levels.
Emerging pathogens
Globalisation has many advantages, but it also provides an opportunity for new and emerging pathogens to spread widely, as demonstrated by the pandemic. There is a need to be kept up to date with clinical knowledge and to develop diagnostic techniques to diagnose these as they arise. The pandemic has shown how adaptable NHS laboratories are at developing ways of working to meet these needs.
Social media and misinformation
Like globalisation, social media has its advantages with regards to healthcare. We can now reach large populations with messages on IPC. However, a downside is the ability to spread misinformation and conspiracy theories that can easily derail public health messages and interventions.
Medical microbiology and virology are specialties that are rapidly learning how to manage misinformation during this pandemic, which has led to vaccine hesitancy. To help tackle some of these misconceptions, the College developed a series of short videos to address some of the most common myths circulating about the COVID vaccines. Medical microbiologists and virologists were involved in the development of scripts and videos.
Sustainability and climate change
The pandemic has highlighted problems around sustainability within the NHS, most notably with disposable personal protective equipment. Many items used in the diagnostic laboratory and in IPC are designated as single use to reduce the incidence of cross-contamination and transmission. However, in the context of climate change, the need to reduce waste and, in particular, plastics pose a challenge and opportunity to use more environmentally friendly resources.
Climate change will also contribute to the emergence of new fungal species that are thermotolerant and pathogenic to humans.
Climate change will also contribute to the emergence of new fungal species that are thermotolerant and pathogenic to humans. These environmental species are often naturally resistant to antifungal agents and survive in a spectrum of environments with the potential to cause hospital outbreaks as described for Candida auris.
Antimicrobial resistance
Antimicrobial resistance is an ever-increasing problem; a report by O’Neill et al. (2014) found that over 50,000 deaths per annum are attributed to antimicrobial-resistant infections across Europe and the USA.3 There has been an increase in antimicrobial consumption, with an almost 40% increase in human consumption between 2000 and 2010.4
Antimicrobial resistance limits the treatment options for often commonly encountered infections. They then become difficult to treat and may be transmitted to other persons. There are very few antimicrobial agents being developed, meaning treatment options may become even more restricted.
In May 2015, the Global Health Assembly adopted a global action plan on antimicrobial resistance, outlining five objectives to help stem the tide of antimicrobial resistance. However, good antimicrobial stewardship at a local and community level is required, not just in healthcare.
Notable people in the specialty
Professor L P Garrod (1895–1979)
Professor Garrod held many posts during his distinguished career, including emeritus professor of bacteriology, University of London, bacteriologist at St Bartholomew's Hospital, London, honorary consultant in chemotherapy to the Royal Post-graduate Medical School at Hammersmith, and consultant in antibiotics to the army. He is best remembered for his work on antimicrobials. He was committed to precise and standardised laboratory testing for antimicrobial resistance, and often advised WHO on this subject.
Professor Garrod published many books and articles and was key in developing guidance on the treatment of subacute bacterial endocarditis as well as demonstrating the harms of prolonged pre-operative antimicrobial prophylaxis. He was heavily involved in the British Medical Journal as author, reviewer, referee, member and Chair of the Journal Committee.
The British Society for Antimicrobial Chemotherapy (BSAC) recognises his incredible achievements and influence on antimicrobial chemotherapy by honouring individuals who are international authorities in this field with the Garrod Medal. The recipient is invited to deliver a lecture (The Garrod Lecture, established 1982) at the BSAC Annual Spring Meeting.
Frederick Sanger (1918–2013)
Biochemist Frederick Sanger is only one of two people to win a Nobel Prize twice in the same category. Although not an infection specialist, his work on genome sequencing has greatly enhanced our understanding of microorganisms and antimicrobial resistance.
In 1964, with Kjeld Marcker, he discovered the formylmethionine tRNA that initiates protein synthesis in bacteria. Three years later, his group had determined the nucleotide sequence of the 5S ribosomal RNA from Escherichia coli, a small RNA of 120 nucleotides. Following these discoveries, he looked at sequencing DNA, which required a different approach to sequencing RNA. In 1975, he and Alan Coulson published a sequencing technique called the plus and minus technique.5 This laborious procedure could sequence up to 80 nucleotides in one go and enabled his group to sequence most of the 5,386 nucleotides of the single-stranded bacteriophage ΦX174, the first fully sequenced DNA-based genome.
In 1977, Sanger and colleagues introduced the Sanger method, utilising a dideoxy chain-termination method for sequencing DNA molecules. This major breakthrough facilitated the rapid and accurate sequencing of long stretches of DNA. It was for this that he was awarded his second Nobel Prize in Chemistry in 1980 (along with Walter Gilbert and Paul Berg). They used this method to sequence human mitochondrial DNA and bacteriophage λ. The same method eventually facilitated the sequencing of the entire human genome.
The Sanger Centre (now the Sanger Institute) was founded by the Wellcome Trust and the Medical Research Council in 1992. It played a key role in the sequencing of the human genome. Although Sanger declined the offer of a knight-hood, not wishing to be seen as different by being known as Sir, he did accept admission into the Order of Merit in 1986, which can only have 24 living members.
Dr Spence Galbraith (1927–2008)
Dr Spence Galbraith was founding Director of the Communicable Disease Surveillance Centre. He was instrumental in utilising information from surveillance, research and outbreak investigations to develop and implement national policy and best practice. After taking up a post as Deputy Assistant Director of Army Health at GHQ, Middle East Land Forces in Egypt in 1953, he developed an interest in the control of communicable diseases. He subsequently spent five years as an epidemiologist in the Epidemiological Research Laboratory at Colindale.
Concerned that the NHS was lacking in epidemiology, Dr Galbraith stated the importance of a nationally coordinated epidemiological service. In January 1977, Dr Galbraith was appointed the first Director of a nationally coordinated epidemiology service, comprising the Communicable Disease Surveillance Centre (CDSC) at Colindale and a number of regional epidemiologists. The government followed his advice, and administered the CDSC by the Public Health Laboratory Service, enabling collaboration between microbiologists and epidemiologists. It is now an integral part of the Centre for Infections within the UK health security agency (UKHSA).
Dr Galbraith is also known for his work on immunisation, developing a national surveillance scheme in 1962 to provide an assessment of the risk of vaccine-associated paralytic polio. He undertook surveillance studies of polio, diphtheria and tetanus, as well as monitoring antibody responses. This allowed him to demonstrate that the Bacillus Calmette–Guérin (BCG), diphtheria/tetanus and oral polio vaccines could be safely and effectively administered to teenagers in one go.
Dr Galbraith was awarded a CBE for his contribution to public health and also the prestigious Harben Gold Medal and lectureship by the Royal Institute of Public Health for ‘eminent services rendered to the public health’. He received the Jenner Medal from the Royal Society of Medicine for distinguished work in epidemiological research.
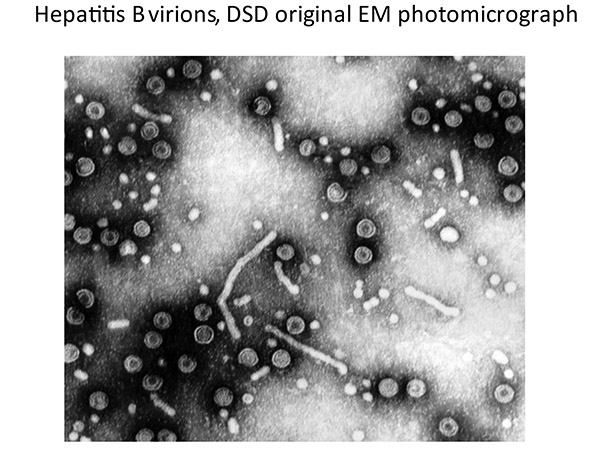
Dr David Dane (1892−1978)
Dr David Dane was a preeminent medically qualified virologist graduating from Clare College Cambridge and qualifying at St Thomas’ Hospital London following a brief interregnum with the SAS (armed forces) in northern France. He moved to the Institute of Medical and Veterinary Science, Adelaide, under an MRC Fellowship as a field virologist investigating zoonotic infections including psittacosis. Returning to Belfast he explored early polio vaccines before becoming Head of Virology at the Middlesex Hospital Medical School.
Dr Dane led the development of the Blood Products Laboratory radioimmunoassay (RIA) for HBsAg for the English blood transfusion service. He went on to lead groundbreaking work in transfusion-transmissible infections, particularly that of hepatitis B and documented sexual transmissibility. His ability to ‘look outside of the box’ was remarkable and had his views on blood safety, entirely applicable in this day and age, been espoused by all, the Infected Blood Inquiry would not be.
He was the first in the UK to use the electron microscope as a routine diagnostic, identifying the 42 nm hepatitis B particle eponymously known as the Dane particle. He supported the development of novel assays (e.g. radial haemolysis and RIAs) as well as conventional tests − from all of which (and his colleagues) he demanded absolute precision. He was a remarkable teacher.
Planning for the future
By working with other infection organisations such as the British Infection Association and Stand-ards for Microbiological Investigations (SMI), the RCPath SAC for Medical Microbiology and Virology is helping its members to develop a workforce that can meet the needs of their local population and maintain a high-quality clinical and diagnostic service, even in the face of a current or future pandemic. This includes increasing the national training numbers in infection training and increasing opportunities for clinical scientists and biomedical scientists to attain leadership and consultant roles within the infection specialty.








